With the development of biotechnology, more and more proteins and polypeptide chains are designed, manufactured and produced through the application of biotechnologies such as genetic engineering, protein engineering and fermentation engineering.
In order to study and apply a certain protein, the target protein must first be separated from the mixed protein molecules and non-protein molecules.
Therefore, the isolation and purification of recombinant proteins is widely used in biochemical research applications and is an important operating technology.
General steps for protein isolation and purification
Material pretreatment and cell disruption
When isolating and purifying a certain protein, it needs to be preprocessed according to the way the protein is expressed. For intracellularly expressed proteins, the protein must first be released from tissues or cells and maintained in its original natural state without losing activity.
Therefore, appropriate methods must be used to disrupt tissues and cells. Commonly used methods for disrupting tissue cells are as follows:
Mortar and pestle
The grinding and wall-breaking method is usually accomplished by placing plant tissue samples in frozen liquid nitrogen and grinding them with a tissue grinder. When tissue material is destroyed, metabolites can be extracted by adding solvent.
Bead grinding method
The bead beating method refers to a method in which glass beads or magnetic beads are added to equal amounts of cell suspension and a vortex mixer to lyse cells through the action of solid shear.
The mechanical shearing force of this cell lysis method is gentle enough to maintain the integrity of organelles. It has a high cell disruption rate and can be used on a large scale. It is suitable for various cell disruptions. The disadvantages are: macromolecular contents are easily deactivated, and slurry separation is difficult.
Ultrasonic crusher
Ultrasonic homogenizers work by inducing vibrations in a titanium probe immersed in a cell solution. A process called cavitation occurs, in which tiny bubbles form and explode, creating localized shock waves and damaging cell walls through pressure changes.
This method works well for plant and fungal cells, but has a drawback: it is noisy, so it must be used in an extra room.
Homogenizer crushing method
The homogenizer uses shear forces on the cells similar to the bead method. Homogenization can be performed by squeezing the cells into tubes that are slightly smaller than they are, thus shearing off the outer layer (French press) or by using a rotating blade in a blender (rotor-stator processor).
Freeze crushing method
Freeze-thaw cycles work by forming ice crystals and cells expanding upon thawing, eventually leading to rupture. For use on algae and soft plant materials. The disadvantage is that it is very time consuming.
High temperature crushing method
High temperatures and pressure can break bonds within cell walls and also denature proteins. While it's fast, if your application is affected by heat damage to the rest of the battery, you'd better find another method.
enzyme
Naturally occurring enzymes can be used to remove cell walls, for example when isolating protoplasts. Depending on the biological tissue sample you are working with, lysozymes such as cellulases, chitinases, lysozymes, mannolases, glycanases, etc. can be used.
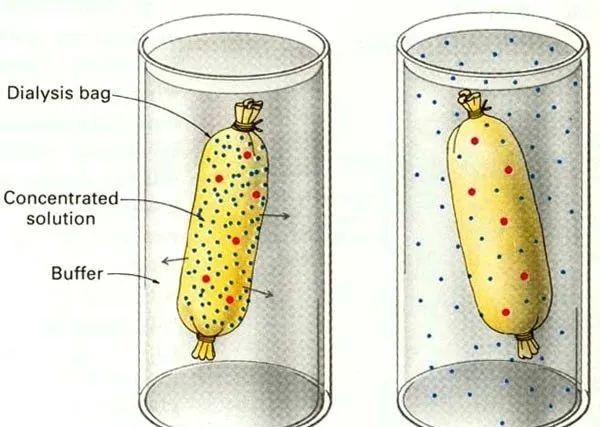
Protein extraction
In the protein extraction process, an appropriate solvent is usually selected to extract the protein. The selection of the composition, pH, ionic strength and other conditions of the buffer solvent used for extraction should be based on the properties of the protein to be prepared.
For example, in the extraction of membrane proteins, surfactants are generally added to the extraction buffer to destroy the membrane structure and facilitate the separation of proteins and membranes. During the extraction process, attention should be paid to the temperature and vigorous stirring should be avoided to prevent protein denaturation. Insoluble matter such as cell debris can be removed by centrifugation or filtration.
Isoelectric precipitation method
Different proteins have different isoelectric points, and they can be separated from each other by isoelectric point precipitation.
Salting out method
Salting out refers to the phenomenon in which neutral salt is added to a protein aqueous solution and the protein precipitates as the salt concentration increases. Salting out is a technique for separating proteins based on their different solubility.
Neutral salt is a strong electrolyte with high solubility. In the protein solution, it competes with the protein for water molecules and destroys the water film on the surface of the protein colloidal particles;
On the other hand, it neutralizes the charges on the protein particles in large quantities, causing the protein particles in the water to accumulate and precipitate. Commonly used neutral salts include ammonium sulfate, sodium chloride, sodium sulfate, etc., but ammonium sulfate is the most common.
The protein obtained by salting out is generally not inactivated and can be re-dissolved under certain conditions. Therefore, this method of precipitating proteins is widely used in the separation, concentration, storage and purification of proteins.
organic solvent precipitation method
Neutral organic solvents such as ethanol and acetone have lower dielectric constants than water. It can reduce the solubility of most globular proteins in aqueous solutions and precipitate them from the solution, so it can be used to precipitate proteins.
In addition, organic solvents will destroy the hydration layer on the protein surface, causing the protein molecules to become unstable and precipitate. Since organic solvents will denature proteins, when using this method, attention should be paid to operating at low temperatures and selecting an appropriate organic solvent concentration.
Further separation and purification of samples
Proteins obtained by isoelectric precipitation and salting out methods generally contain other protein impurities, which require further separation and purification to obtain samples with a certain degree of purity. Commonly used purification methods include: gel filtration chromatography, ion exchange cellulose chromatography, affinity chromatography, etc. Sometimes it is necessary to use a combination of these methods to obtain higher purity protein samples.
gel filtration chromatography
Gel filtration chromatography, also known as exclusion chromatography or molecular sieve method, mainly separates and purifies proteins based on their size and shape, that is, their quality.
The packing in the chromatography column is some inert porous network structure material, mostly cross-linked polysaccharide material, which allows the substances in the protein mixture to be separated according to different molecular sizes, and the macromolecular substances are excluded from the outside and flow down. The distance is short and flows out first, while small molecular substances can enter the interior of the chromatography packing and have a longer distance when flowing down, so they flow out later.
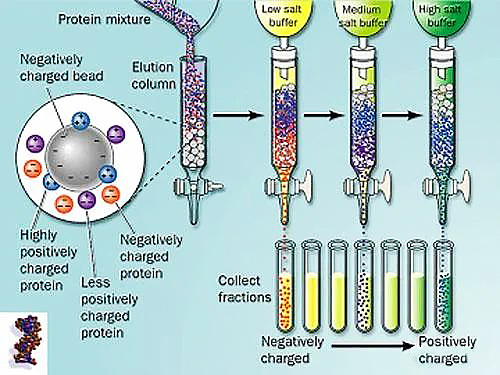
Ion exchange chromatography
Ion exchange chromatography is a separation method based on the different isoelectric points of proteins. When the proteins are under different pH conditions, the proteins have different charges.
In ion exchange chromatography, the matrix is composed of charged resin or cellulose. Those with positive charges are called anion exchange resins; those with negative charges are called cation exchange resins. Commonly used ion exchangers for protein separation include weakly acidic carboxymethylcellulose and weakly alkaline diethylaminoethylcellulose.
Hydrophobic interaction chromatography
Hydrophobic chromatography is also called chromatography under hydrophobic interaction. From the perspective of separation and purification mechanism, it also belongs to the category of adsorption chromatography.
Hydrophobic chromatography uses hydrophobic ligands coupled to a stationary phase carrier to reversibly combine with some hydrophobic molecules in the mobile phase for separation. Protein surfaces generally have hydrophobic and hydrophilic groups. Hydrophobic chromatography utilizes the hydrophobicity of a certain part of the protein surface to bind to a hydrophobic carrier at high salt concentrations.
During elution, the salt concentration is gradually reduced. Due to their different hydrophobicity, they are eluted and purified one by one. It can be used to separate proteins that are difficult to purify by other methods.
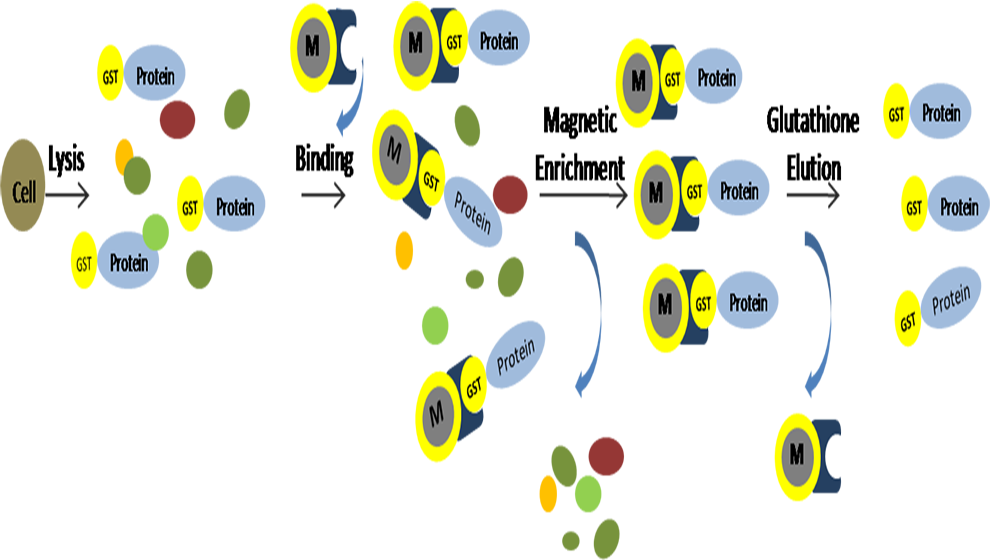
Affinity chromatography
Affinity chromatography is a chromatographic method that uses specific interactions between biomolecules to separate molecules.
Affinity chromatography links molecules that have a certain binding ability to the substance to be separated on a gel filtration chromatography column, and their binding is reversible, and the two can be separated from each other when the mobile phase conditions are changed.
Affinity chromatography can be used to purify or concentrate a molecule from a mixture, or it can be used to remove or reduce the content of a molecule in a mixture.
Conclusion
Protein separation and purification technology is a key step in post-fermentation treatment. The rationality of the operation directly affects the final yield of the product, and is directly linked to the company's production costs and profits. Proficiently mastering protein separation and purification technology and using reasonable methods to improve purity while ensuring yield are eternal topics worthy of study in post-fermentation treatment.